Welcome!
The Swedish Consortium for Artificial Photosynthesis is a collaborative research environment with the purpose of advancing the science and utilization of solar fuels - fuel from solar energy. We bring together leading scientists with expertise in a broad range of disciplines within chemistry, physics and biology. Together we do fundamental and applied research, developing solutions for a sustainable future.
News
Gustav Berggren, doubly awarded
Long-standing CAP member and Professor Gustav Berggren has been awarded the 2024 Hugo Theorell Prize in Biophysics "for his strong commitment to integrating the disciplines of biophysics, biochemistry, and chemistry, into the studies of gas processing metalloenzymes." Read the interview with Gustav on the Swedish Chemical Society webpage.
Gustav has also been awarded the EuroBIC medal, celebrated in the closing ceremony of the 2024 edition of the biannual conference EuroBIC. This prestigious award recognizes outstanding young scientists who are conducting innovative research in the field of Bioinorganic Chemistry within European laboratories and actively contributing to the European Bioinorganic Chemistry community.
Warm congratulatons Gustav!
Princess R. Cabotaje receives global recognition
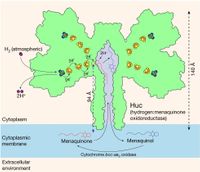
Princess R. Cabotaje, currently doing her PhD in the group of Gustav Berggren, was selected as one of the three finalists in the annual 3-minute thesis competition. In her presentation, she explained her research on rare NiFe hydrogenases that not only tolerate oxygen, but are also capable of extracting the trace amounts of hydrogen gas present in our atmosphere, thereby providing the bacteria with energy and seemingly allowing them to live on air. Though she did not win the competition, Princess won global recognition as a video of her performance, posted on social media, went viral.
Further reading: "Structural basis for bacterial energy extraction from atmospheric hydrogen."
How water splits during photosynthesis
The final and crucial step of the water oxidation mechanism in Photosystem II has been investigated by the research group of Johannes Messinger in collaboration with groups at Lawrence Berkeley National Laboratory and others, using serial femtosecond x-ray crystallography. The work, which was published in Nature, will lead to a more complete understanding of the underlying mechanism of photosynthetic water oxidation, and offers inspiration for the development of water-splitting technologies to produce solar fuels.
Read the research paper in Nature, and the accompanying News&Views article.
How microorganisms extract hydrogen from the atmosphere
A biological method for extracting hydrogen from the atmosphere has been identified by members of the Gustav Berggren group, together with researchers from Australia and the USA, and published in Nature. The interdisciplinary group of researchers have isolated and mapped a unique variant of a nickel-iron hydrogenase that not only withstands oxygen, but is also capable of extracting trace amounts of hydrogen gas present in the atmosphere, thereby supplying the bacterium with energy.
Read the news article from Uppsala university (in Swedish) or go directly to the Nature article.
CAP at the meeting venue Haga Slott, Enköping, in September 2022.
Partners:
©CAP 2025