Welcome!
The Swedish Consortium for Artificial Photosynthesis is a collaborative research environment with the purpose of advancing the science and utilization of solar fuels - fuel from solar energy. We bring together leading scientists with expertise in a broad range of disciplines within chemistry, physics and biology. Together we do fundamental and applied research, developing solutions for a sustainable future.
News
Event summary: Uppsala University conference on Sunlight- and Power-to-X
On October 24-26, 2023, in a collaboration with SUNERGY, CAP organized a conference on research and innovation on sustainable fossil-free fuels and base chemicals.
The conference was attended by more than 170 people: researchers, company representatives, policymakers, and other interested parties. These three days were aimed at accelerating the development of technology for producing solar fuels, electro-fuels, and sustainable base chemicals, with a focus on technologies for direct H2 production and CO2 valorization from renewable energy.
Nobel prize in chemistry for nanotechnology with relevance to artificial photosynthesis!
This year's Nobel Prize in chemistry has been awarded to Moungi Bawendi, Louis Brus and Alexei Ekimov “for the discovery and synthesis of quantum dots.” In CAP, we are particularly happy for this, as this fundamental science is highly relevant to solar energy conversion and the field of solar fuels research. Two of CAP's prominent researchers are active in related fields, Jacinto Sá who works on plasmonic materials, and Haining Tian who develops polymer dots, somewhat related to quantum dots. CAP extends its congratulations to the Nobel Laureates!
Read more about the Nobel Prize here.
October 24 - 26: Uppsala University conference on Sunlight- and Power-to-X
Eva von Bahr Lecture Hall, Uppsala university, Ångström laboratory
This October, we organize a 2-day conference on research and innovation for sustainable fossil-free fuels and base chemicals by direct H2 production and CO2 valorization from renewable energy. We bring together researchers, companies, policymakers, and other stakeholders, to accelerate the development of solar fuels, electro-fuels, and sustainable base chemicals in Sweden and in Europe. PhD students and postdocs are welcome to present their work in the poster exhibition.
For programme and registration, follow this link!
Stefano Crespi awarded the Gustafsson prize!
The Göran Gustafsson prize in physics is awarded to the CAP member Stefano Crespi. His research is focused on the design of new photochemical reactions and to find new ways to utilize light stimuli and convert them into mechanical motion on the nanometer scale, to design new light-driven molecular machines. The prize is a research grant of 2,75 MSEK.
Read more on the home page of the Göran Gustafssons stiftelse (Swedish).
How water splits during photosynthesis
The final and crucial step of the water oxidation mechanism in Photosystem II has been investigated by the research group of Johannes Messinger in collaboration with groups at Lawrence Berkeley National Laboratory and others, using serial femtosecond x-ray crystallography. The work, which was published in Nature, will lead to a more complete understanding of the underlying mechanism of photosynthetic water oxidation, and offers inspiration for the development of water-splitting technologies to produce solar fuels.
Read the research paper in Nature, and the accompanying News&Views article.
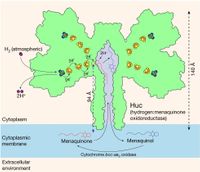
How microorganisms extract hydrogen from the atmosphere
A biological method for extracting hydrogen from the atmosphere has been identified by members of the Gustav Berggren group, together with researchers from Australia and the USA, and published in Nature. The interdisciplinary group of researchers have isolated and mapped a unique variant of a nickel-iron hydrogenase that not only withstands oxygen, but is also capable of extracting trace amounts of hydrogen gas present in the atmosphere, thereby supplying the bacterium with energy.
Read the news article from Uppsala university (in Swedish) or go directly to the Nature article.
The 2022 CAP workshop was a success!
In 2022, on September 27-28, enthusiastic CAP members gathered at Haga Slott in Enköping for two half-days of intense discussions, idea hatching, future shaping, and fun.
Images from the 2022 CAP workshop.
Peafowl Plasmonics raises 40 MSEK
In seed round led by Industrifonden and the Wallenberg fund Navigare Ventures, the startup company Peafowl Plasmonics, founded by the CAP member Jacinto Sá, has raised enough capital to take the company to the next level.
Read about it here (in Swedish): Peafowl Plasmonics har tagit fram genomskinliga solceller (di.se)
The CAP workshop, September 27-28, 2022:
CAP at the meeting venue Haga Slott, Enköping, in September 2022.
Partners:
©CAP 2023